Our Services
ISO 13485
-----------------
Certification for Medical Device Manufacturers
What is ISO 13485
The ISO 13485 standard is for manufacturers of medical devices or medical device components. Due to the vital nature of products manufactured for medical use, product conformity of medical devices is paramount.
The global acceptance of ISO 13485:2016 helps safeguard product conformity as manufacturing supply chains have expanded nationally and internationally. For an overview of ISO 13485 download the free information sheet.
Safeguard product conformity!
The global acceptance of ISO 13485:2016 helps safeguard product conformity as manufacturing supply chains have expanded nationally and internationally. For an overview of ISO 13485 download the free information sheet.
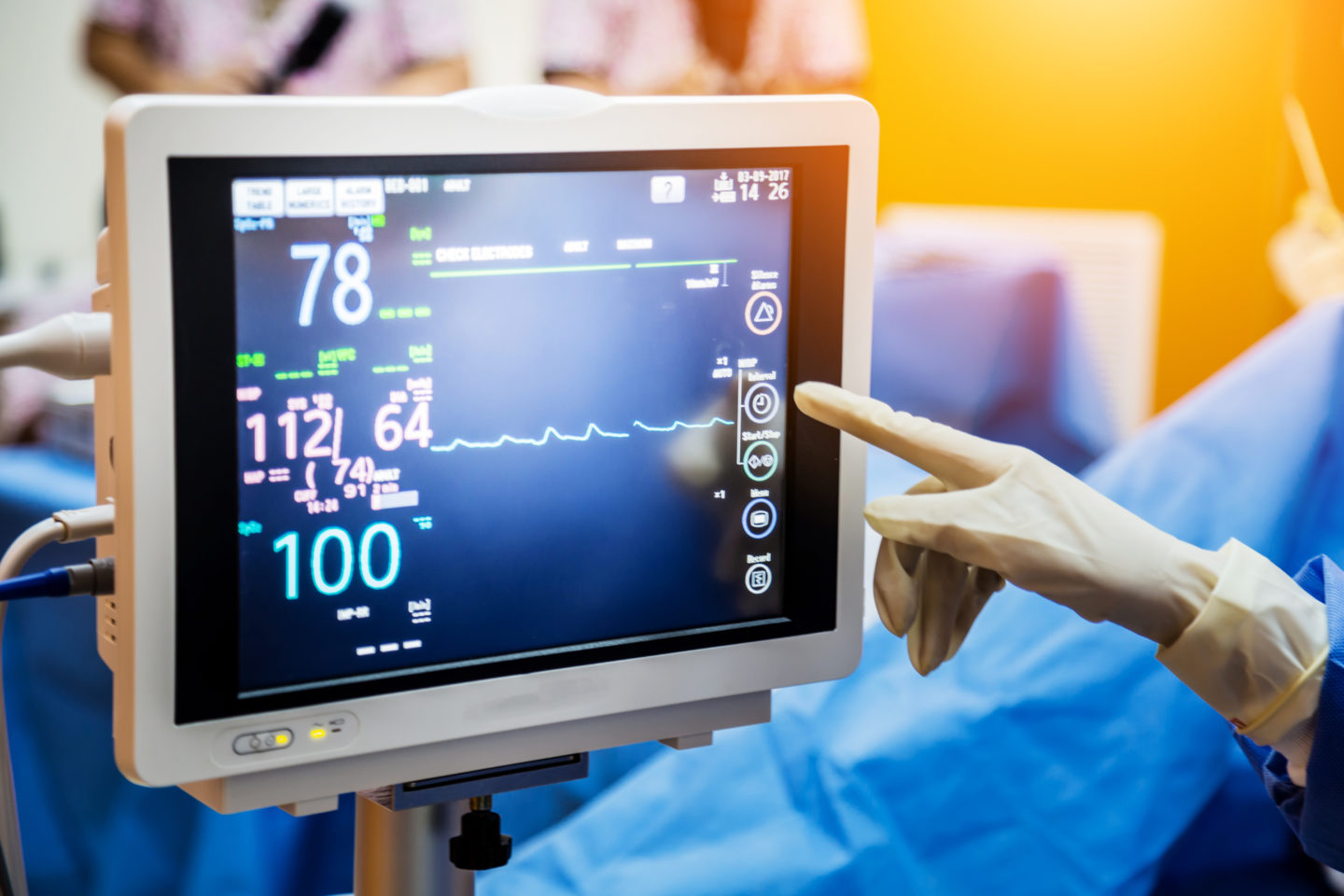
Let’s face it, safety and quality are paramount in the medical device industry. Whenever we go to the doctor or hospital or have a procedure, we assume as consumers that every device being used is safe and well tested. The medical device industry is under FDA Regulatory requirements in the United States.
Product safety & on-time delivery!
This should give all of us peace of mind; however, as a medical device manufacturer, you have a responsibility and a requirement to consistently deliver devices that are high quality, safe and effective. The medical device industry is made up of many different and complex regulations, standards and other requirements.

RPM CONSULTING INC.
ISO 13485 Compliance


The processes for design and manufacturing of medical devices is the subject of this standard. It references the ISO 9001:2008 standard with extensive additions pertaining to more stringent controls applicable to such sensitive products. In many countries, statutory and regulatory requirements are based on the ISO 9001 and ISO 13485 standards. An example is the U.S. revised Quality System Regulation (FDA 21 CFR 820) which in large part incorporates these two ISO international standards.
ISO - 13485
GET CERTIFIED

ISO 13485 Certification Benefits
Small businesses may gain a number of benefits from an ISO 13485:2016 certification:
• Foundation for establishing compliance with FDA, MDD or CE requirements.
• Ensure QMS practices that produce consistently safe and effective medical devices
• Helps you manage risk effectively
• Provides for the opportunity to improve QMS processes and efficiencies
• Creates opportunities to gain new business within medical device industries
• Essential controls to ensure product conformity and regulatory compliance
The new ISO 13485:2016 standard emphasizes risk management and risk-based decision making for processes outside the realm of product realization.

Consulting Support for ISO 13485
The focus is on safety and performance of medical devices and compliance with regulatory requirements.
ISO 13485:2016 is a stand-alone standard. It is similar in scope and intent to ISO 9001:2008.
RPM Consulting, Inc. has qualified ISO 13485 consultants ready to help you achieve certification. Our approach can be achieved simply and affordably for small businesses with our expert consultants.
RPM Consulting, Inc. provides consulting support for the ISO 13485 standard through Remote/Online and Onsite support programs.
We also provide consulting support for companies seeking multiple certifications (such as ISO 9001, AS 9100, ISO 14001, ISO 45001 and IATF 16949) through an Integrated Management System.
Our consultants translate the technical language of the ISO 13485 Standard and make it as simple and effective for your organization as possible.
ISO 13485:2016 certification is commonly an initial step for medical device manufacturers seeking compliance with the requirements of the Medical Device Directive (MDD) and the CE marking process in the European Union (EU).
Complying to ISDO 13485 is your step toward meeting these requirements.
4 Simple Steps to Certification:
Step 1: Plan — As we assist you in learning the basics of the ISO requirements, your consultant will perform a Gap Analysis, if required, help you prepare the required documentation, quality manual, documented processes.
Step 2: Implement — your consultant will assist your company as you implement the ISO process, working with your team to set goals, conduct management reviews, review results, and drive the action needed for improvement.
Step 3: Review — An full system internal audit will be conducted to ensure you are prepared for the registrar audit. Together, you will review process corrections that are needed and make sure you have all the necessary documentation you will need to be in compliance with the standard.
Step 4: Certify — Finally, RPM Consulting, Inc. will assist you in securing a registrar, or Certification Body, to perform your final Stage 2 external audit. Upon completion, we will assist you to correct any findings (if applicable), then you will receive your ISO certificate.
